25 Dec 2025
- 12 Comments
When you’re diagnosed with hormone receptor-positive breast cancer, your doctor will likely mention hormone therapy. It’s not chemotherapy. It doesn’t kill cells outright. Instead, it starves the cancer by blocking the hormones that feed it. For many women, this is the most important treatment after surgery - and it can last for years. Two drugs dominate this space: tamoxifen and aromatase inhibitors. They work differently, have different side effects, and aren’t right for everyone. Choosing between them isn’t just about which is stronger - it’s about your body, your life, and what you’re willing to live with.
How Hormone Therapy Stops Breast Cancer
Not all breast cancers are the same. About 8 in 10 are hormone receptor-positive, meaning they grow when estrogen sticks to them. Hormone therapy doesn’t touch the tumor directly. It cuts off the fuel. That’s it. And that’s why it works so well over time.
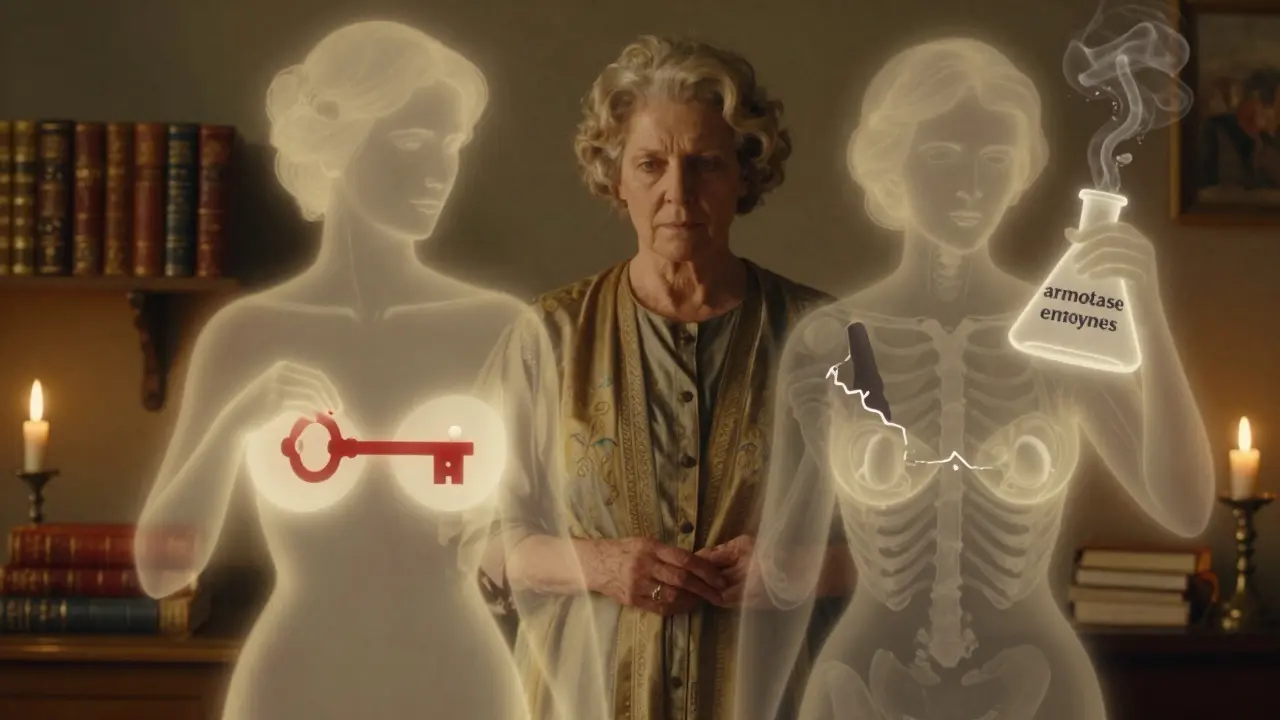
Tamoxifen acts like a fake key. It slips into the estrogen lock on cancer cells, but it doesn’t turn the lock. No estrogen gets in. No growth signal. It’s called a selective estrogen receptor modulator, or SERM. It blocks estrogen in the breast but can act like estrogen elsewhere - like in bones and the uterus. That’s why it helps protect bone density but raises the risk of uterine changes.
Aromatase inhibitors (AIs) are different. They don’t block estrogen receptors. They stop estrogen from being made at all. In postmenopausal women, most estrogen comes from fat tissue, not ovaries. The aromatase enzyme turns male hormones into estrogen. AIs shut that down. Anastrozole, letrozole, and exemestane reduce estrogen levels by 95% or more. That’s powerful. But it also means your whole body is low on estrogen - not just the breast.
Who Gets Tamoxifen - And Who Gets Aromatase Inhibitors
This isn’t a one-size-fits-all choice. Menopause status is the biggest factor.
If you’re premenopausal - your ovaries are still making estrogen - tamoxifen is the standard. AIs won’t work unless you also shut down your ovaries. That’s a whole other layer: injections like goserelin to stop ovarian function. It’s effective, but it means sudden menopause - hot flashes, sleep loss, mood swings, and bone thinning all at once. For many young women, tamoxifen alone is easier to live with.
For postmenopausal women, AIs are now the first choice. Data from over 30,000 women in the EBCTCG meta-analysis showed AIs reduce recurrence risk by 30% more than tamoxifen during treatment. Ten-year breast cancer death rates were lower too - 12.1% with AIs versus 14.2% with tamoxifen. That’s not a small difference. It’s the difference between living and dying.
But here’s the catch: tamoxifen still has a role. If you can’t tolerate AIs, or if your cancer is low-risk, tamoxifen is perfectly valid. And for some women, switching from tamoxifen to an AI after 2-3 years gives the same benefit as starting with an AI. That’s called sequential therapy. It’s an option if side effects are too tough early on.
The Side Effect Battle: What You’ll Actually Feel
Doctors talk about recurrence risk. Patients talk about joint pain.
Aromatase inhibitors are notorious for musculoskeletal issues. Half of women on AIs report joint stiffness or pain - sometimes so bad they can’t hold a coffee cup. In one survey, 22% of AI users quit treatment because of it. Bone density drops faster too. About 6.4% of women on AIs broke a bone over 10 years, compared to 5.1% on tamoxifen. That’s why DEXA scans every 1-2 years are standard. If your bone score falls below -2.0, you’ll likely get a drug like denosumab or zoledronic acid to protect your skeleton.
Tamoxifen doesn’t cause joint pain like AIs. But it has its own burdens. Hot flashes? Almost universal. Night sweats? Common. And then there’s the uterus. Tamoxifen can cause thickening of the uterine lining. That raises the risk of endometrial cancer - 1.2% over 10 years versus 0.4% with AIs. It’s rare, but it’s real. That’s why any abnormal bleeding after menopause needs to be checked immediately.
There’s also blood clots. Tamoxifen increases the risk of pulmonary embolism - a clot in the lung - by about 2-fold compared to AIs. That’s why women with a history of clots or stroke usually avoid it.
And then there’s brain fog. Both drugs can cause it, but in online communities, women on AIs report it more often. One Reddit analysis found nearly half of AI users said their memory or focus worsened. Tamoxifen users mostly complained about hot flashes - 63% of them.

What the Experts Really Say
Dr. Paul Goss, who led the MA.17 trial on letrozole, says AIs give a 30% improvement in disease-free survival for postmenopausal women. But he also stresses bone health management. You can’t just prescribe the drug and walk away.
Dr. Meredith Regan, who worked on the TEXT and SOFT trials, points out that for high-risk premenopausal women, adding exemestane with ovarian suppression prevents one extra recurrence for every 31 women treated. That’s a powerful number. But it’s not for everyone. If your cancer is low-risk, tamoxifen alone is still the safer, simpler choice.
Dr. V. Craig Jordan - the scientist who helped develop tamoxifen - warns against over-relying on AIs. He argues tamoxifen’s benefits to bone and heart health in younger women might balance out its slightly higher recurrence risk. For some, it’s not about the numbers. It’s about living well.
ASCO’s 2022 guidelines say it plainly: “Patient values should guide selection.” If you hate joint pain more than you fear a recurrence, tamoxifen might be right. If you’re terrified of cancer coming back and can handle the stiffness, AIs are stronger.
How Long Do You Take It?
Five years used to be the standard. Now, it’s more flexible.
For average-risk patients, 5 years is enough. But if your cancer had high-risk features - large tumor, positive lymph nodes, high grade - you might go to 7, 8, or even 10 years. The MA.17X and DATA trials showed that extending therapy beyond 5 years cuts recurrence risk even further.
For premenopausal women on tamoxifen, 10 years is now recommended by some guidelines. The NSABP B-14 trial showed long-term tamoxifen cuts late recurrences - the kind that pop up 5, 10, even 15 years later.
And here’s something new: the PERSEPHONE trial is testing whether 3 years of tamoxifen works just as well as 5 for low-risk patients. If it does, it could change practice overnight. Fewer side effects. Less cost. Same protection.

What’s Next? The Future of Hormone Therapy
The field isn’t standing still. In 2023, the FDA approved camizestrant, a new oral SERD - a selective estrogen receptor degrader. Unlike tamoxifen, which blocks estrogen, SERDs destroy the estrogen receptor entirely. Early data shows a 38% reduction in recurrence for women with ESR1 mutations - a subtype that often resists AIs.
There’s also pharmacogenomics. Tamoxifen needs to be turned into endoxifen by the liver enzyme CYP2D6. Some people have a genetic variant that makes them slow metabolizers. They don’t make enough endoxifen. A 2012 JAMA study found these women had 2.5 times higher recurrence risk. The CYRILLUS trial is now testing whether testing for this gene can guide dosing - maybe giving higher tamoxifen to slow metabolizers.
And then there’s access. In the U.S., branded AIs cost $150 a month. Tamoxifen? $15. In low-income countries, tamoxifen is often the only option. It’s cheap, stable, and effective. The global fight isn’t just about science - it’s about equity.
Real Decisions, Real Lives
One woman I spoke with - 54, postmenopausal, stage II, node-negative - chose tamoxifen over an AI because her mother had a hip fracture at 72. She didn’t want to risk hers. Another - 47, high-risk, BRCA1-negative - switched from tamoxifen to exemestane after 3 years because her Oncotype DX score was 28. She wanted every advantage.
There’s no perfect choice. Only the right one for you.
Some women take their pills for 10 years without a hiccup. Others can’t make it past year two. It’s not weakness. It’s biology. And it’s okay to stop. Your doctor won’t judge you. They’ll help you find another path.
What matters most isn’t which drug you pick. It’s that you take it. Hormone therapy doesn’t work if you skip doses. It doesn’t work if you quit because the joint pain is too much. It works because you keep going - even when it’s hard.
And if you’re unsure? Ask for a second opinion. Ask for a pharmacist consult. Ask for support groups. You’re not alone. And you’re not just a patient. You’re the one who decides what your life looks like after cancer.
Is tamoxifen still used today for breast cancer?
Yes, tamoxifen is still widely used - especially for premenopausal women. It’s also used in men with breast cancer and in women who can’t tolerate aromatase inhibitors. For low-risk postmenopausal women, tamoxifen remains a valid option. It’s not outdated - it’s a tool, and it’s still effective.
Can you switch from tamoxifen to an aromatase inhibitor?
Yes, many women switch after 2-3 years of tamoxifen. Studies show this approach gives the same long-term benefit as starting with an AI. It’s often done if side effects like hot flashes are too strong early on, and the woman is postmenopausal. Your doctor will check your hormone levels to confirm menopause before switching.
Do aromatase inhibitors cause weight gain?
Weight gain isn’t directly caused by aromatase inhibitors, but it’s common. Lower estrogen levels can slow metabolism and increase fat storage, especially around the abdomen. Many women gain 5-10 pounds over 5 years. It’s not inevitable - regular exercise and a protein-rich diet help. But it’s something to expect and plan for.
How do I know if I’m a CYP2D6 poor metabolizer?
You can get tested with a simple saliva or blood test. The test looks at your CYP2D6 gene to see how well your body turns tamoxifen into its active form, endoxifen. If you’re a poor metabolizer, your doctor might consider a higher dose of tamoxifen or switch you to an aromatase inhibitor. Testing isn’t routine yet, but it’s becoming more common in specialized cancer centers.
Are there natural alternatives to tamoxifen or aromatase inhibitors?
No. Supplements like turmeric, flaxseed, or soy may have weak estrogen effects, but they are not proven to prevent recurrence. Relying on them instead of prescribed hormone therapy increases your risk of cancer coming back. Always talk to your oncologist before taking anything new - even something labeled “natural.”
What happens if I stop hormone therapy early?
Stopping early increases your risk of recurrence - especially in the first 5 years. The risk goes up the sooner you stop. If you’re struggling with side effects, don’t quit without talking to your doctor. There are ways to manage them - lower doses, timing changes, medications for joint pain, or switching drugs. But stopping without a plan is dangerous.
Can men take tamoxifen or aromatase inhibitors for breast cancer?
Yes. About 1% of breast cancers occur in men, and nearly all are hormone receptor-positive. Tamoxifen is the standard treatment for men. Aromatase inhibitors are rarely used because men produce estrogen differently - they need ovarian suppression to make AIs work, which isn’t possible. Tamoxifen is effective and well-tolerated in men.

wendy parrales fong
December 26, 2025Just wanted to say this post made me feel seen. I was on tamoxifen for 7 years and honestly? The hot flashes were brutal, but I kept going because I didn’t want to be one of those stats.
It’s not about being strong. It’s about showing up for yourself, even on the days you just want to crawl under the covers.
Thank you for writing this.
Jeanette Jeffrey
December 26, 2025Oh please. Everyone’s acting like this is some profound life decision. It’s just pharmacology. AIs reduce recurrence by 30%? Big whoop. If you’re too weak to handle joint pain, maybe you shouldn’t have gotten cancer in the first place.
Also, tamoxifen causes uterine cancer? Wow. Groundbreaking. Next you’ll tell me smoking causes lung disease.
Shreyash Gupta
December 26, 2025bro why are we even debating this 🤡
if u got the cash go with AI. if u got no money? tamoxifen. simple.
also can we talk about how 90% of this data is from white women??
what about us in the global south? we don’t even get DEXA scans 😭
Ellie Stretshberry
December 26, 2025i had the ai and the joint pain was real like i couldnt open jars or hug my kid without wincing
my dr said switch to tamoxifen and honestly it felt like a weight lifted
the hot flashes? yeah they suck but at least i could hold a coffee cup again
also i cried reading the part about taking it for 10 years
youre not alone
Zina Constantin
December 27, 2025This is one of the most thoughtful, comprehensive explanations of hormone therapy I’ve ever read. The balance between clinical data and human experience is rare.
Thank you for highlighting that this isn’t about choosing the ‘best’ drug - it’s about choosing the right one for *your* life.
Also, kudos for mentioning access disparities. Tamoxifen’s $15 price tag isn’t just a footnote - it’s a lifeline for millions.
Dan Alatepe
December 27, 2025yo i read this whole thing at 3am after my third night of insomnia from the AI
my hips feel like they’re full of gravel
but i saw my niece’s first steps yesterday and i’m still here
so yeah i’ll take the pain
also my aunt died from breast cancer in 2010 and she never even got tamoxifen
so i’m not complaining
just saying
Angela Spagnolo
December 29, 2025...I just... I didn’t realize how much I needed to hear this.
I stopped tamoxifen at 18 months because the night sweats were making me feel like I was burning alive...
and I’ve felt so guilty ever since.
But you said it’s okay to stop...
...and that you won’t judge...
...I’m crying.
Thank you.
Sarah Holmes
December 31, 2025How dare you suggest that tamoxifen is a 'valid option' for postmenopausal women? The data is unequivocal - aromatase inhibitors are superior in every measurable metric. To recommend anything less is irresponsible medical advice disguised as 'patient empowerment.'
And don’t get me started on the dangerous myth that 'natural alternatives' can substitute for proven therapy. This is cancer, not a yoga retreat.
It’s not about 'feelings.' It’s about survival.
Jay Ara
December 31, 2025my mom took tamoxifen for 10 years and never missed a pill
she said it was like brushing her teeth
just do it
even on bad days
you got this
and if you need help with the joint pain
ask for physical therapy
it helped her
Michael Bond
January 2, 2026AI for postmenopausal. Tamoxifen for pre. That’s it.
Kuldipsinh Rathod
January 3, 2026my cousin switched from tamoxifen to an AI after 2 years and said the change was like going from a foggy room to sunlight
but she also took magnesium and did yoga
and the joint pain got better
maybe it’s not just the drug
maybe it’s how you live with it
SHAKTI BHARDWAJ
January 3, 2026you think this is hard? try being a woman in india with no insurance and a husband who says 'why are you wasting money on pills when you're already sick?'
my sister took tamoxifen for 2 years and then stopped because she couldn't afford the follow-up scans
she died at 42
and now you're all here debating which drug is 'better'
some of us don't get to choose
we just survive